The temperature of air or any other gas depends exclusively on the speed of its molecules. The air molecules are always in motion. Thus, when we cool or heat rooms in buildings, we change the speed of the air molecules in the rooms. When we cool a room, we slow down the air molecules, when we heat a room, we speed up the air molecules.
To change the temperature or humidity in a room, or to add oxygen O2 to a room, or to remove carbon dioxide CO2 from a room, we use this behavior of the air molecules.
For example, when we atomize some perfume in the room, the molecules leave the flacon. They spread throughout the room by their own movement and the movements of the surrounding molecules without any ventilation.
When we use the molecular movement of air (natural diffusion or Brownian motion) for cooling, heating, supply and removal of gas components, we copy a system from nature. Insects have been using it to breathe for about 480 million years. Nature shows us that this principle works.
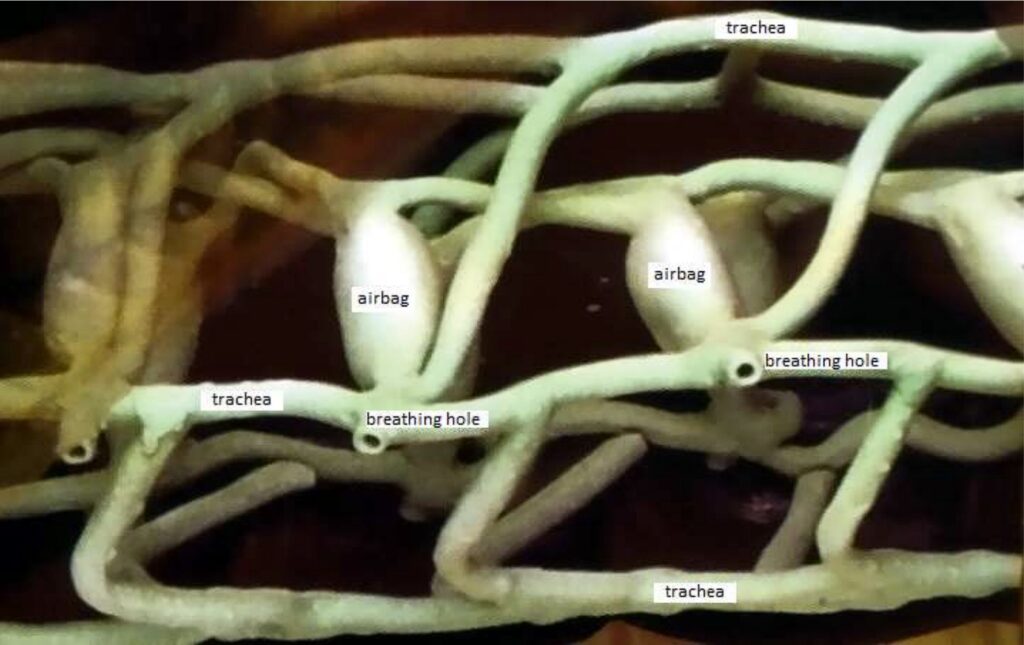
For example, insects don’t have lungs like we do. They breathe with a system of tubes and capillaries that branch out from the outside of the body to the muscles. Through this system, the muscle tissue is directly connected to the outside air.
Only through the natural diffusion the oxygen O2 moves to the muscles and the CO2 from the muscles to the outside. With this system, oxygen is distributed throughout the insect’s body.
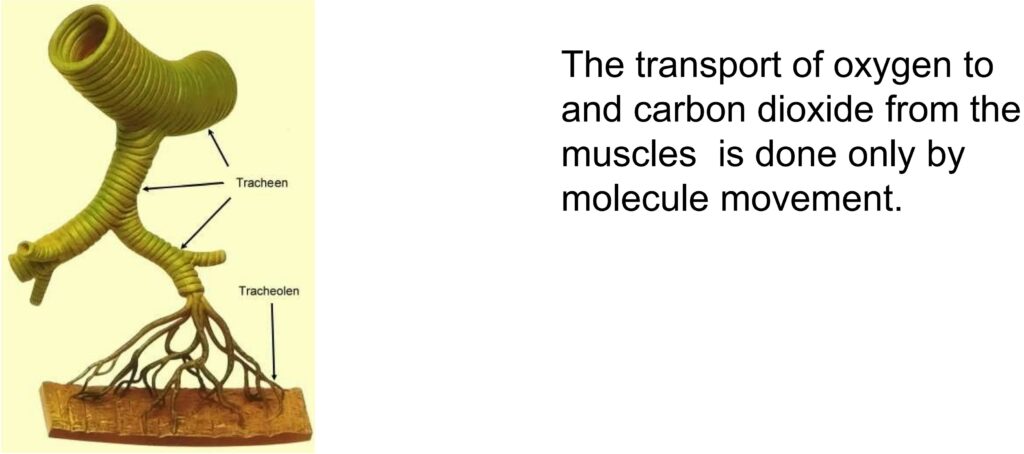
This is caused by the molecular movement of the air (which we copy from nature).
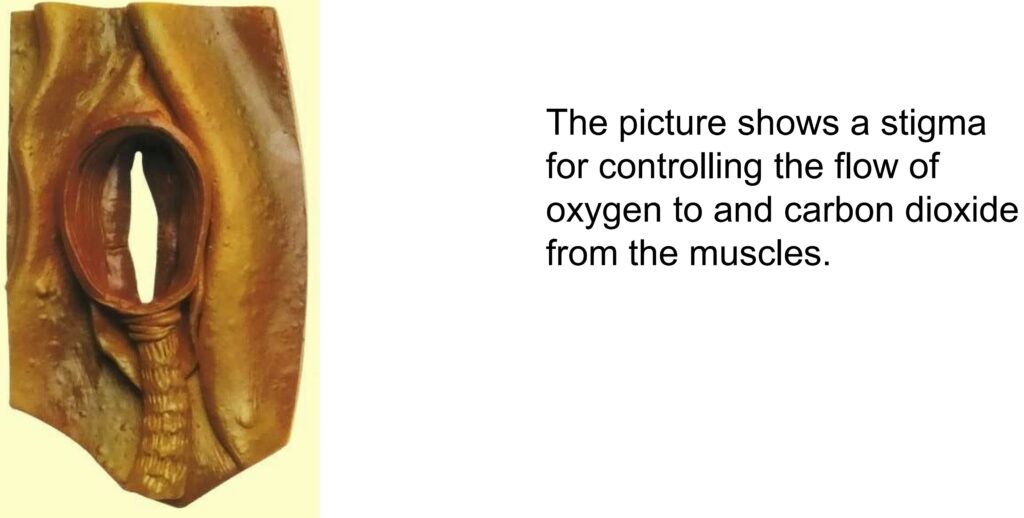
An insect can thus supply its muscles with 100 times more oxygen per unit of time than we can with our lung breathing.